[2025-09-26] (Open access) Your 2025 training plan is not yet complete? Good news: you can still take action right now!
Discover our INTER-enterprise training calendar for September, October and November, or ask us about our INTRA-enterprise on-site training sessions. We also offer e-learning courses on MDR and IVDR, with continuous access to content.
Whether you’re a manufacturer or a subcontractor, these sessions will help you better understand and apply the regulatory and standards requirements for medical devices.
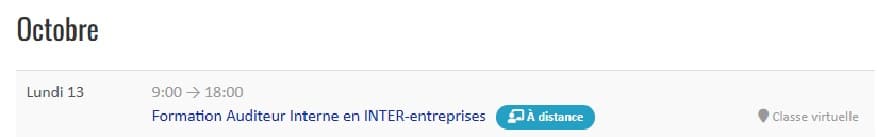
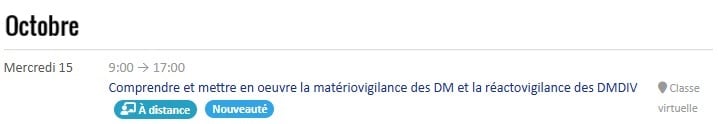
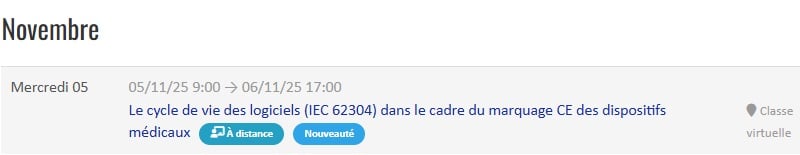
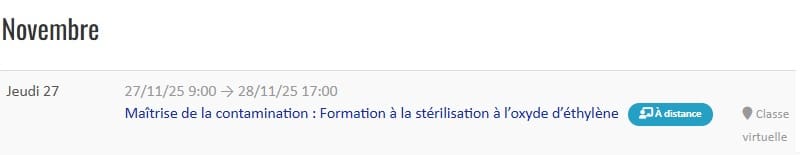
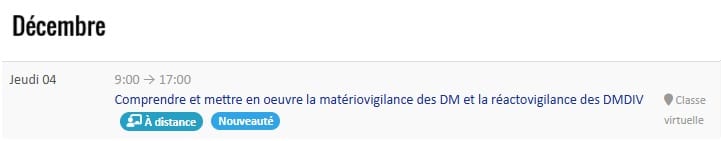
Our not-to-be-missed INTER-company training courses
DMEXPERTS: INTER- or INTRA-company training courses for medical devices
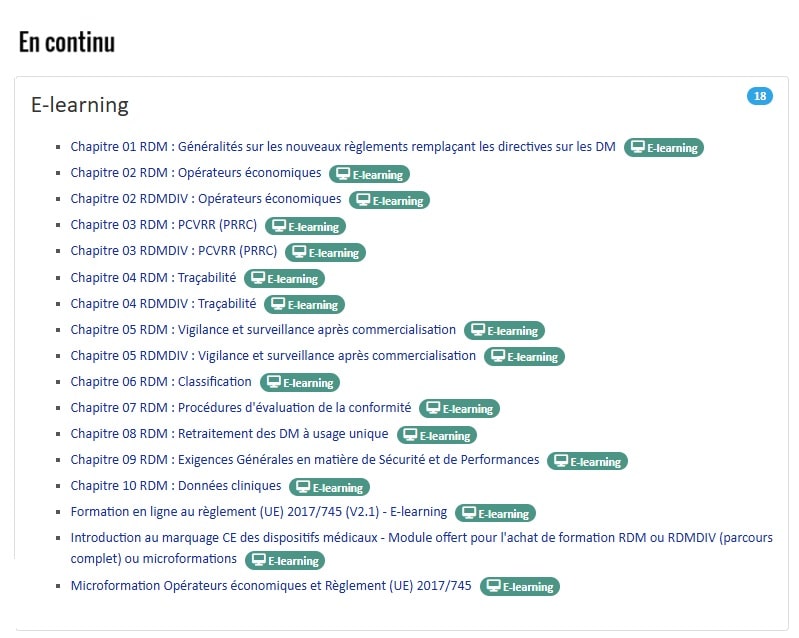
Our e-learning courses
DMEXPERTS: MDR and IVDR e-learning courses, available in less than 10 minutes
Why choose DMEXPERTS?
✔ Senior expertise: training courses designed by experts, combining theory and answers to your practical questions for direct application in your company.
✔ Flexible formats: face-to-face or distance learning according to your preferences.
✔ INTER-company sessions: ideal for sharing experiences and exchanging ideas with other industry professionals.
✔ INTRA-company sessions: customized to your products and/or quality system, with possible adjustment of content and duration to suit your needs.
To find out more, visit our training catalog to talk to our team and organize your training courses.
TOGETHER, WE’LL SUCCEED!